You may be interested in
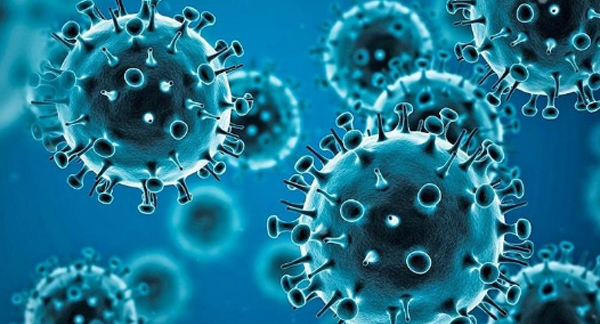
COVID-19 Vaccination Programs & Implications
Of The Human Microbiome

Diabetes & Endocrinology

Cardiology
Neurology

Oncology & Haematology

Dermatology
Pulmonology & Respiratory

Gastroenterology

Rheumatology

Obstetrics & Gynaecology & Womens Health Care

Ophthalmology

Extensive experience in clinical research, we possess a comprehensive understanding of the drug development process

Ready with resources to cater for the ever expanding requirement(s) of clients
Team is agile and adapts quickly, able to pivot and change course
Automation (online) of activities EDC, Data capturing, Patient IVRS & Analytical activities

Team is combine in-depth therapeutic knowledge with a passion for clinical research

Our cost control mechanisms guarantee a positive cost-benefit without compromising the quality of data and compliance